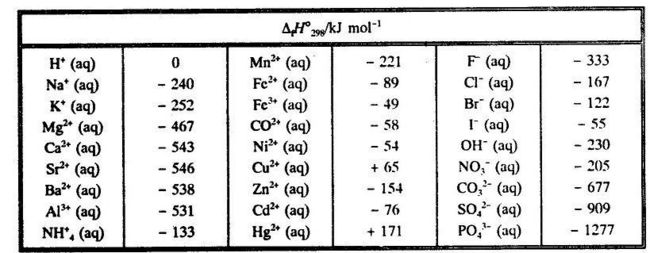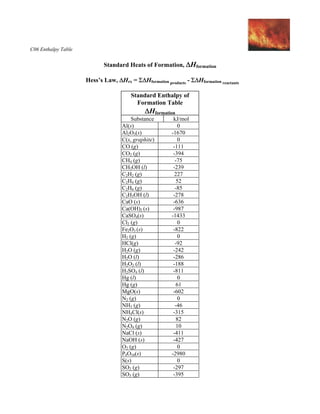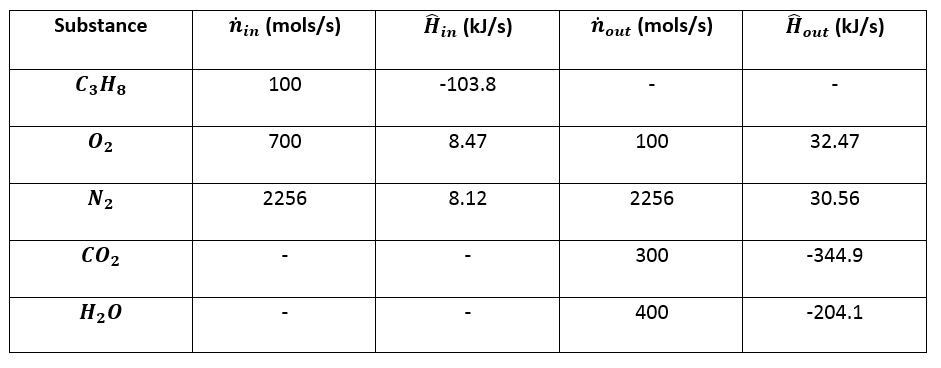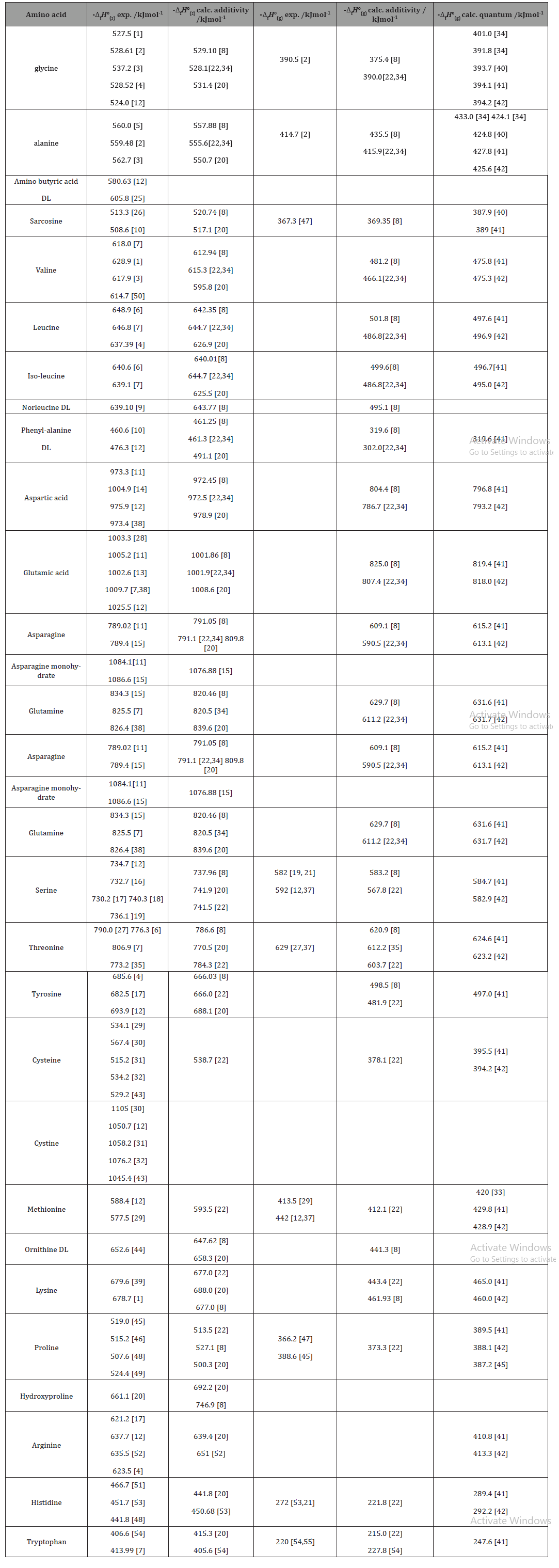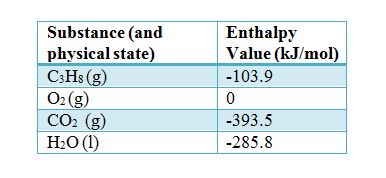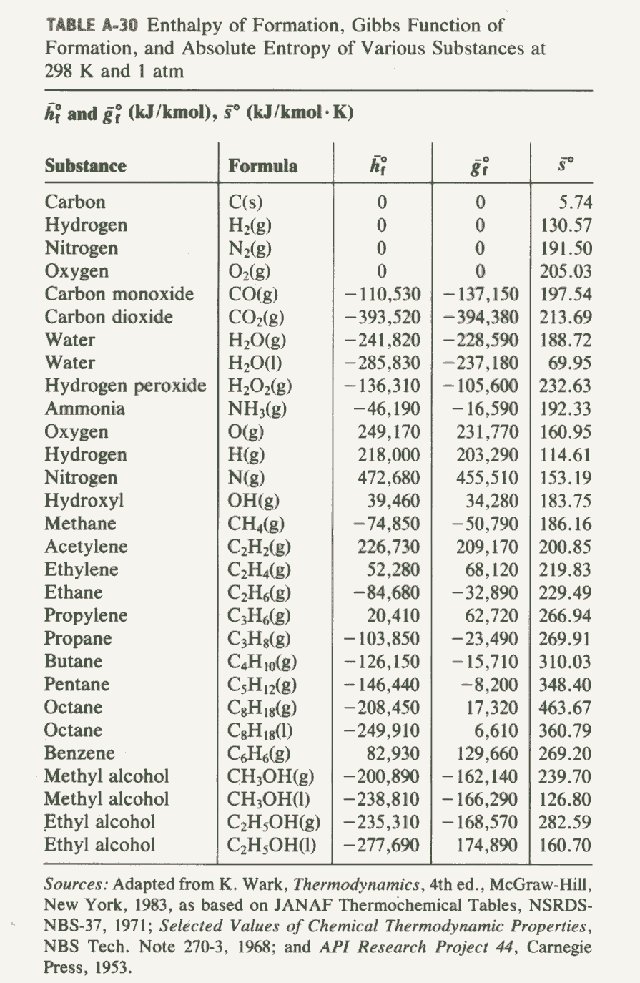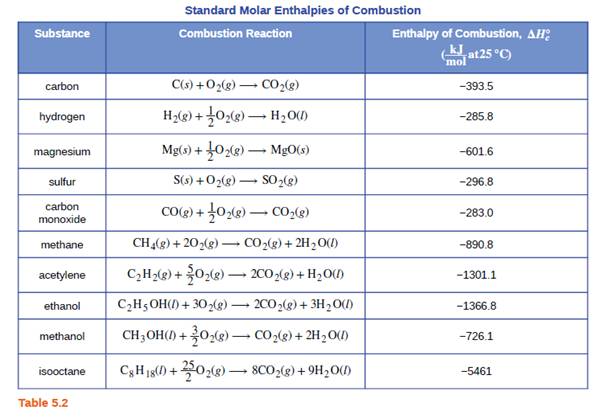
Which of the enthalpies of combustion in Table 5.2 the table are also standard enthalpies of formation? | bartleby

Solved) - The value for the standard heat of combustion, ?H° combustion, for... (1 Answer) | Transtutors
Calculate the enthalpy of reaction for the reaction "CH"_3"COOH" + "H"_2"O" -> "CH"_3"CH"_2"OH" + "O"_2? | Socratic

Table 6 from Structure and heats of formation of iodine fluorides and the respective closed-shell ions from CCSD(T) electronic structure calculations and reliable prediction of the steric activity of the free-valence electron
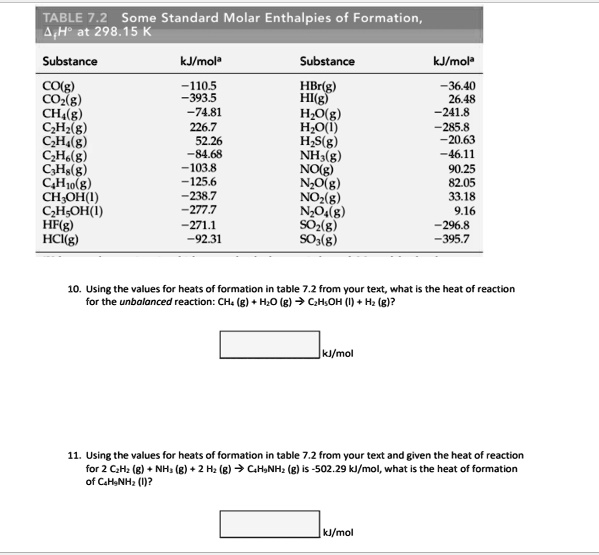
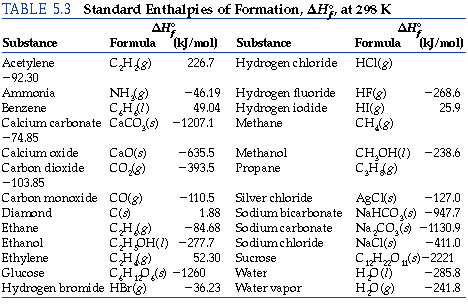
SOLVED: TABLE 7.2 Some Standard Molar Enthalpies of Formation; Heat at 298.15 K Substance kJ/mol Substance kJ/mol CO(g) CO2(g) CH4(g) C2H6(g) C3H8(g) C4H10(g) C6H6(g) C6H12(g) C6H14(g) C6H6OH(l) C2H5OH(l) -105 -393.5 -74.81 226.7

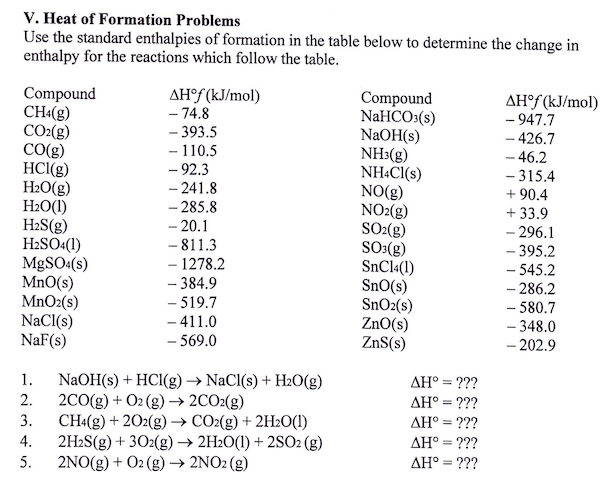
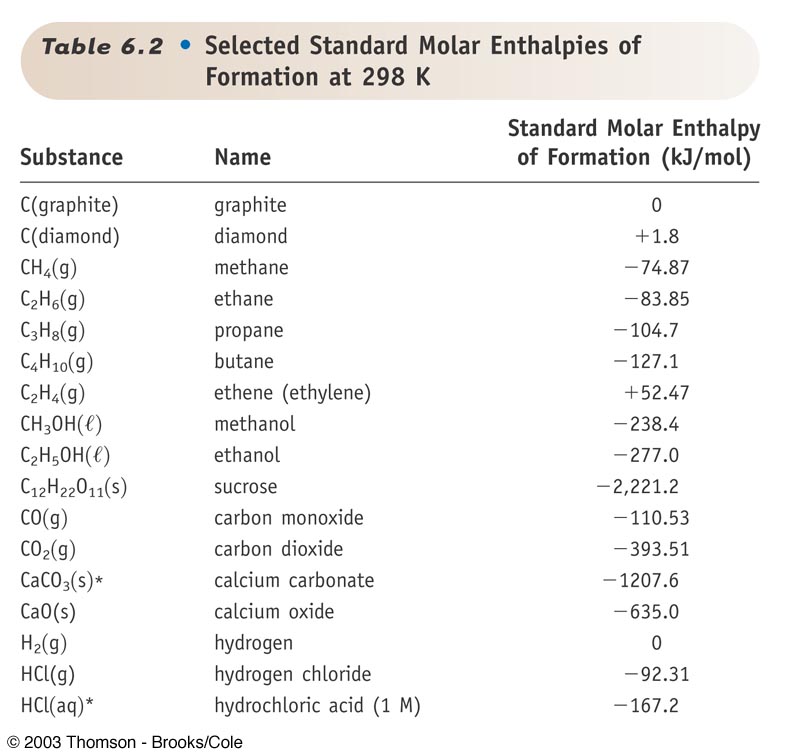
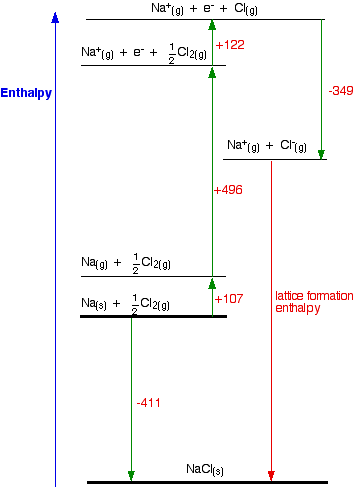
:max_bytes(150000):strip_icc()/GettyImages-154953454-6806780a2f0f4ec99daf580619b5aeef.jpg)